<div>
Ammonium Formate,Ammonium Formate manufacturer in India, Ammonium Formate Applications, Uses of Ammonium Formate, Storage of Ammonium Formate, Chemical Formula of Ammonium Formate, Ammonium Formate Characteristics, Benefits of Ammonium Formate, Ammonium Formate Uses in Industry, Handling Ammonium Formate, Ammonium Formate Composition
<h2>Applications of Ammonium Formate</h2>
<p>Ammonium formate (NH4HCO2) is a versatile compound used extensively across several industries. Its primary applications include catalytic reduction reactions and as a buffer in high-performance liquid chromatography (HPLC). In the chemical industry, ammonium formate is crucial for palladium on carbon (Pd/C) reduction and reductive amination processes.</p>
<h2>Ammonium Formate Properties</h2>
<p>Ammonium formate is a white, crystalline powder that is both hygroscopic and colorless. It possesses unique chemical properties that make it suitable for various industrial applications. The powder’s ability to form formamide upon heating, while decomposing into formic acid, underscores its utility in chemical processes and storage.</p>
<h2>Ammonium Formate Uses</h2>
<p>The uses of ammonium formate extend beyond basic applications. In organic synthesis, it serves as a key reagent for the Leuckart reaction, facilitating the reductive amination of aldehydes and ketones. Additionally, its role as a buffer in HPLC helps in stabilizing the pH of solutions, which is crucial for accurate chromatographic analysis.</p>
<h2>Ammonium Formate Chemical Formula</h2>
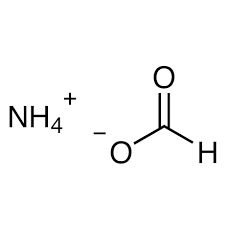
<p>The chemical formula for ammonium formate is NH4HCO2. This formula represents the ammonium salt of formic acid, which is integral in various industrial processes and chemical reactions. Understanding its chemical structure helps in comprehending its behavior and applications.</p>
<h2>Ammonium Formate Decomposition</h2>
<p>When heated, ammonium formate undergoes decomposition, losing water elements and forming formamide. Further decomposition yields hydrogen cyanide (HCN) and water (H2O). This decomposition process is significant in chemical reactions where formamide is a desired product.</p>
<h2>Ammonium Formate Production</h2>
<p>As a premier manufacturer, we focus on the production of high-purity ammonium formate. The production process involves using the finest raw materials and advanced techniques to ensure that the final product meets the highest standards of quality and effectiveness.</p>
<h2>Ammonium Formate for HPLC</h2>
<p>In high-performance liquid chromatography (HPLC), ammonium formate is used as a buffering agent. Its ability to maintain stable pH levels ensures precise and reliable chromatographic analysis. This makes it a preferred choice for analytical labs and industries requiring accurate separation and detection of compounds.</p>
<h2>Ammonium Formate in Organic Synthesis</h2>
<p>In organic synthesis, ammonium formate plays a critical role, especially in the reductive amination of aldehydes and ketones. The Leuckart reaction, which utilizes ammonium formate, is essential for producing various amines and other organic compounds.</p>
<h3>High-Purity Ammonium Formate</h3>
<p>We specialize in providing high-purity ammonium formate, which is essential for applications requiring stringent quality standards. Our product is tested rigorously to ensure that it meets the required purity levels, making it suitable for critical industrial and analytical uses.</p>
<h3>Ammonium Formate Safety Data</h3>
<p>Safety is paramount in handling ammonium formate. We provide comprehensive safety data sheets (SDS) to inform users about proper handling, storage, and emergency measures. This ensures that our clients can use the product safely and effectively in their operations.</p>
<h3>Ammonium Formate Analysis</h3>
<p>Accurate analysis of ammonium formate is crucial for its effective application. Our quality control measures include detailed analysis to ensure that the product complies with the specified standards and meets the required chemical and physical properties.</p>
<h3>Ammonium Formate Reduction Reactions</h3>
<p>Ammonium formate is used in reduction reactions, particularly with palladium on carbon (Pd/C). This application is vital for reducing various functional groups and is widely used in the pharmaceutical and chemical industries.</p>
<h4>Ammonium Formate Storage</h4>
<p>Proper storage of ammonium formate is essential to maintain its quality and effectiveness. It should be stored in a cool, dry place, away from moisture and direct sunlight, to prevent any degradation or loss of its properties.</p>
<h5>Ammonium Formate Properties and Specifications</h5>
<p>Our ammonium formate complies with stringent specifications to ensure it meets industry standards. Key properties include its white crystalline appearance, hygroscopic nature, and specific pH range in aqueous solutions.</p>
<h6>Ammonium Formate Buffer Solutions</h6>
<p>In analytical chemistry, ammonium formate is used to prepare buffer solutions that maintain stable pH levels. This application is crucial for accurate and reliable results in various analytical techniques.</p>
</div>
We offer premium quality products manufactured using the finest raw material available and purest chemical. Pure ammonium formate decomposes into formamide and water when heated which is its primary use in industry. Since ammonium formate is also produced from formic acid, it serves as a way of storing formic acid.
It is the ammonium salt of formic acid with chemical formula NH4HCO2. It is a colorless, hygroscopic and crystalline solid. When heated, ammonium formate loses the elements of water, forming formamide. Upon further decomposition this decomposes to HCN and H2O.
Various Applications of Ammonium Formate:
- It is used in palladium on carbon (Pd/C) reduction
- It is used for reductive amination of aldehydes and ketones (Leuckart reaction)
- It is used as a buffer in high-performance liquid chromatography (HPLC)
DescriptionWhite crystalline Powder hygroscopicCompliesPasses pH (5%Aqueous. solution)Between 6 to 76.80PassesWater ContentNot More Than 5% w/w3.5%PassesAssay By Potentiometer on Anhydrous basisNLT 97%97.16 %Passes
| Product Details |
| Name of the Product |
Ammonium Formate |
| CAS Number |
540-69-21 |
| Description |
White crystalline powder hygroscopic |
| PH (5%Aqueous. solution) |
Between 6 to 7 |
| Water Content |
Not More Than 5% w/w |
| Assay by potentiometer on anhydrous basis |
96.90% |
Results of Analysis and Protocols of the Test applied
| TEST |
LIMIT |
RESULT |
| Characteristics |
Colourless water like liquid |
O.K |
| Boiling range |
95 % material distills between range of 51 and 53 Deg Celsius |
O.K |
| Specific Gravity at 25 Deg Celsius |
Between 1.104 to 1.11 |
1.105 |
| Assay |
Not less than 99 % |
99.25 % |